Importance of Pharmaceutical Artwork Management in 2025
Feb 28, 2025
Imagine spending a decade and millions of dollars developing a breakthrough drug, only to have a tiny packaging error derail the launch.
It’s a nightmare scenario: a misplaced decimal point on a label or a mistake in one of the language translations forces an expensive product recall.
In the pharmaceutical industry, “artwork” refers to all the printed packaging components – labels, cartons, blister pack designs, inserts, etc. – that convey essential information about a medicine.
In short, pharmaceutical artwork management is both vital and challenging – and getting it right is non-negotiable for any drug manufacturer.
Importance of Pharmaceutical Artwork Management
Why invest time and resources in streamlining artwork management? Because the stakes are incredibly high. Here are some of the key reasons this discipline is so important:
1. Regulatory Compliance
Drug packaging and labeling must meet strict guidelines set by regulators in every market. Every word, format, and detail on a label is scrutinized for compliance with regulations and current Good Manufacturing Practices (cGMP).
Failure to comply can result in rejected submissions, fines, or forced product recalls.
2. Patient Safety
Accurate labeling is directly tied to patient health. A clear and correct label helps healthcare providers and patients use the medicine safely.
Conversely, a small error or omission on packaging can lead to medication errors with serious consequences.
For example, confusing or incorrect dosage information could cause an overdose or underdose, and mistranslating a warning could put patients at risk.
3. Brand Consistency
Pharmaceutical companies rely on consumer and prescriber trust, and packaging is a big part of that trust.
Consistent branding across all products and markets – from the logo and colors to the tone of text – reinforces credibility.
A well-managed artwork process enforces standardized templates, style guides, and branding rules so that every package reflects the company’s identity correctly.
This consistency not only strengthens brand image but also prevents confusion that can arise if different batches or regions had noticeably inconsistent packaging.
4. Efficiency and Cost Savings
Modernizing artwork management also brings significant operational benefits.
A streamlined process with fewer manual touchpoints means faster approvals and product launches, which can be a competitive advantage. It also prevents costly mistakes.
Packaging and labeling errors are a leading cause of product recalls, accounting for over 50% of recalls in the pharma industry.
Every recall or redesign due to artwork mistakes costs money (in wasted stock, logistics, and damage control) and delays time-to-market.

How to Conduct Effective Pharmaceutical Artwork Management
So, how can drug manufacturers manage packaging artwork in a way that ensures accuracy and compliance without grinding the product launch process to a halt?
Below are the key steps to conduct it successfully:
1. Initiate Artwork Requests and Gather Data
Every artwork cycle should start with a clear, formal request.
Identify why a change or new artwork is needed – for instance, is it a new product launch, a formulation change, a regulatory update, or a correction of an error?
Use a standardized Artwork Change Request form to document the scope and reason for the change.
Gather all necessary data at this stage (such as product details, dosing information, marketing text, and regulatory references) so that the team has a single source of truth.
It’s wise to have an initial quality review (often by a QA or regulatory specialist) of the request to assess its impact on patient safety and compliance before proceeding.
2. Collaborate Across Departments
Pharmaceutical artwork development is a team sport that spans multiple departments and even external partners.
To be effective, you need to break down silos and involve all relevant stakeholders early.
This typically includes:
Regulatory Affairs (for content accuracy and legal compliance),
Quality Assurance (for error checking),
Marketing/Branding (for design and brand alignment),
Supply Chain/Manufacturing (for print feasibility and scheduling), and often
External design or printing vendors.
All these players must be able to communicate and pass data back and forth seamlessly, otherwise the process will bog down.
3. Ensure Regulatory Compliance at Every Step
Built into your process should be checkpoints for regulatory compliance.
As artwork is created or updated, it must be vetted against the relevant rules and guidelines for each target market.
This means verifying that all required information is present (correct drug name, strength, dosage form, usage instructions, warnings, storage conditions, manufacturer details, etc.) and formatted according to regulations.
If you operate globally, be aware of differing requirements: what passes in one country might need tweaking in another (e.g. font sizes, barcode formats, or the inclusion of QR codes per new guidelines).
Having a compliance review built into the workflow (and ideally, automated checks – more on that later) will mitigate the risk of a costly redo or a non-compliance issue.
4. Manage Localization and Translations
Most pharmaceutical products are sold in multiple countries, which means artwork often needs localization into various languages and regional formats.
Effective artwork management involves coordinating with translators or in-country reviewers who can produce accurate translated text for labels and leaflets.
Each translation should be carefully verified for correctness (a mistranslated dosage or warning could be disastrous).
Additionally, layout adjustments might be required to accommodate different text lengths or legal statements in each language.
Managing this can be complex, so a best practice is to maintain a database of approved translations and use controlled templates where possible.
Meticulous version control here is vital – you need to keep track of which language versions have been updated and approved.
5. Quality Assurance and Proofreading
Before any artwork gets the final green light, it must pass through rigorous quality checks. Adopting a “four-eye principle” – where at least two qualified people review every artwork change – is a common practice to catch mistakes others might miss.
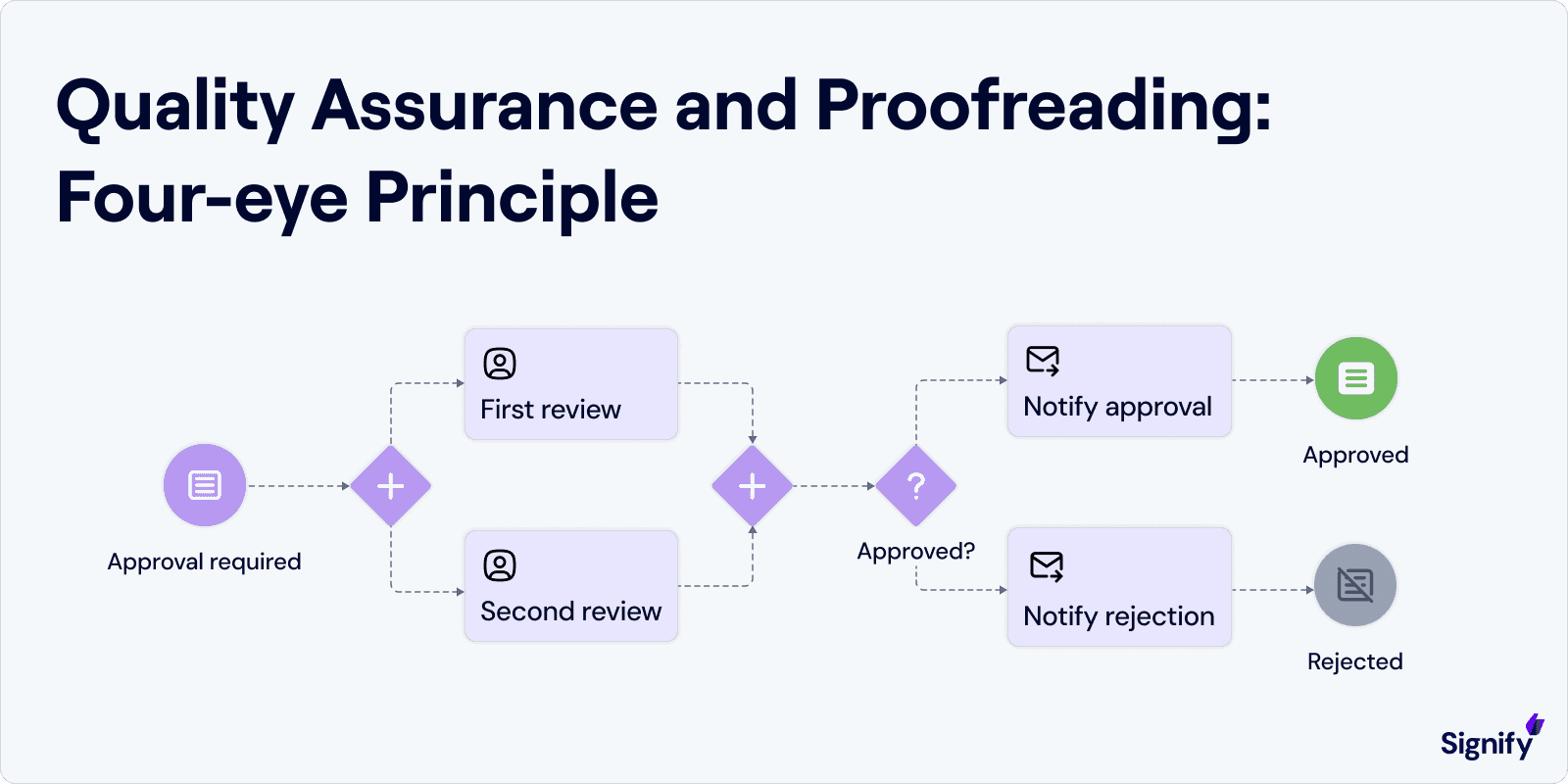
Proofreading should compare the artwork against the original source text (like the approved labeling text or Product Information) line by line.
Many pharma companies now use electronic proofreading tools to assist in this process.
These software tools can do things like pixel-level comparisons (to catch any unintended font or layout shifts), text comparisons (to ensure no words or numbers deviated from the source), barcode verification, Braille checks, and color accuracy checks.
Every change cycle should also be carefully documented – if a reviewer marks an annotation (e.g., “change 5mg to 50mg”), the next proofing pass should confirm that it was indeed changed.
6. Documentation, Version Control, and Audit Trail
An often overlooked but absolutely essential part of artwork management is maintaining proper documentation and version control throughout the process.
In a compliant system, every version of an artwork (draft v1, v2, final, etc.) should be saved and traceable, and every approval or change should be logged.
This creates an audit trail that shows who reviewed and approved what and when.
Not only is this good project management, but it’s typically required by regulators – auditors may ask to see evidence of the approval process and changes to a product’s packaging.
With Simplify, you can:
Automate Evidence Traceability – Instantly locate critical compliance information with advanced document extraction, eliminating manual searches.
Enhance Accuracy & Efficiency – Quickly identify and reference key sections, reducing errors and saving valuable time.
Ensure Seamless Audit Trails – Maintain thorough documentation with effortless tracking, making compliance audits stress-free.
Having this organized pays off in multiple ways: it provides traceability in case of an audit or investigation, it helps train new team members on what was done and why, and it creates institutional knowledge for future artwork changes.
Best Practices for Streamlining Artwork Management
Implementing the above steps is a great start, but how can we make the artwork process truly efficient and “right-first-time”?
Below are some best practices drug manufacturers should consider to further streamline artwork management:
Standardize Workflows and Templates
One common pitfall is ad-hoc or inconsistent processes across products or departments.
It’s far more efficient to have a standardized workflow for artwork changes – with clearly defined stages, responsible persons, and expected turnaround times for each step.
For example, use a standardized checklist or procedure every time an artwork change is initiated so nothing is overlooked.

Similarly, standardize the artwork templates and style guides your teams use.
Templates for packaging components (with predefined font styles, placeholder text for required fields, barcodes, etc.) ensure a consistent look and compliance format, reducing the chance of missing information.
Companies that rely too much on manual, case-by-case processes often see more errors; disconnected files and fragmented communications can lead to someone working off the wrong version or skipping an approval.
By contrast, a well-designed standard process makes it repeatable and predictable – everyone knows the drill, and artwork moves from draft to print in a controlled way.

Leverage Digital Tools and Automation
Modern packaging artwork management can benefit greatly from digital solutions.
If you’re still managing revisions via endless email chains and spreadsheets, upgrading to an Artwork Management System (AMS) or similar digital workflow tool can be a game-changer.
These systems centralize the process and often come with automation features: for instance, automated notifications to reviewers, templates for different product types, and even automated content checks.
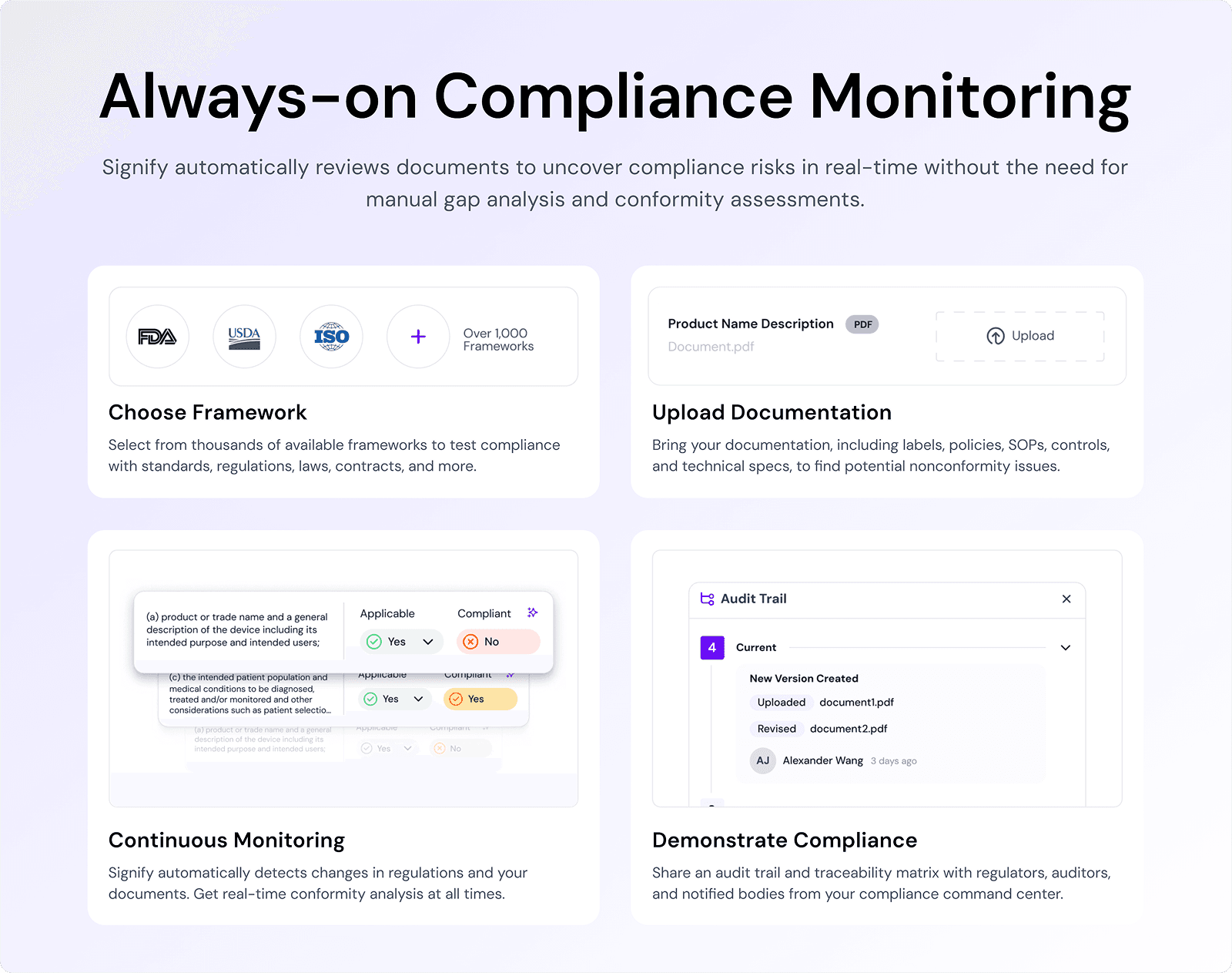
Reducing manual tasks means fewer opportunities for human error.
Proofreading software (as mentioned earlier) automatically detects discrepancies far more efficiently than a purely manual review.
Likewise, comparison tools can ensure that barcode data or serial numbers on artwork match the source data. The result is a significant drop in errors and revision cycles.
Keep Up with Regulatory Changes
The regulatory landscape for pharmaceutical labeling is not static – guidelines are frequently updated to improve patient safety, incorporate new technologies (like electronic labeling or QR codes), or harmonize across regions.
A best practice for artwork management is to have a mechanism to monitor regulatory changes and swiftly implement necessary updates to your templates or content.
What can you do?
Subscribe to updates from agencies (FDA, EMA, etc.),
Participate in industry forums, or
Have regulatory intelligence tools like Signify.
For example, if the FDA introduces a new requirement for pediatric dosing on labels, you should proactively update your artwork checklists and training so that all new artworks comply.
Keeping labeling content in sync with the latest regulations requires continuous adaptation.
Companies should conduct periodic reviews of their artwork against current guidelines to catch any needed changes early (rather than during a panicked scramble when a deadline looms).
In practice, this might involve quarterly audits of a sample of product artworks for compliance or maintaining a matrix of requirements by country that is regularly updated and consulted during the artwork creation.
Conclusion
Pharmaceutical artwork management might not grab headlines, but it is a mission-critical function that underpins compliance, patient safety, and operational efficiency in drug manufacturing.
As we’ve discussed, managing packaging artwork effectively ensures that medicines are delivered with the right information every time – which means patients and healthcare providers can trust what they see on the label.
It also means manufacturers stay on the right side of regulators and avoid the costly nightmares of recalls or enforcement actions.
For drug manufacturers reading this, the call to action is simple: take a hard look at your current artwork processes and tools. Are they meeting today’s challenges of global compliance and speed? If not, it’s time to invest in modern artwork management systems and process improvements.
Signify: Elevate Artwork Compliance with Smart Automation
Dramatically cut down on labeling errors, shipment holds, and manual workflows. Signify seamlessly integrates with your artwork management process, automatically checking every label against key regulations like the FD&C Act and the Fair Packaging and Labeling Act.
Our AI-powered platform instantly flags potential issues, giving you real-time updates and clear action steps so you can launch products with full confidence.
Real-Time Updates & Streamlined Workflows
Why juggle endless spreadsheets and out-of-date SOPs? Signify continuously tracks global regulatory changes, instantly updating your compliance checklists and shipping documents.
By automating tedious tasks, your team can focus on innovation instead of paperwork, driving faster approvals and smoother market entry.
Reduce Complexity in Highly Regulated Markets
Whether you’re shipping locally or worldwide, Signify verifies that labels meet every country’s laws and certifications, shielding you from costly delays and regulatory fines.
Our powerful risk notifications and continuous monitoring turn complicated compliance processes into a straightforward, accelerated workflow.
Ready to Simplify compliance?
Discover how Signify can transform your artwork management and supercharge your time to market.
Book a Demo today to see how easy compliance can be!
