IEC 62304 Checklist: Key Points and Best Practices
Aug 21, 2024
As the medical device industry continues to evolve and innovate, software has become an increasingly critical component of many devices. With this growing reliance on software comes the need for rigorous quality control and regulatory compliance. One of the key standards medical device manufacturers must adhere to is IEC 62304 - the international standard for medical device software lifecycle processes.
In this comprehensive guide, we'll walk through an essential IEC 62304 checklist to help ensure your medical device software is audit-ready and compliant. We'll cover the key elements of the standard, discuss common pitfalls, and provide actionable tips for implementing an effective compliance management system.
Why IEC 62304 Compliance Matters
Before diving into the checklist, it's important to understand why IEC 62304 compliance is so crucial for medical device manufacturers:
Regulatory requirements: IEC 62304 is recognized by major regulatory bodies, including the FDA and EU notified bodies. Compliance is often necessary for market approval.
Risk mitigation: The standard helps identify and mitigate potential software-related risks, enhancing patient safety.
Quality assurance: By following IEC 62304 processes, manufacturers can improve overall software quality and reliability.
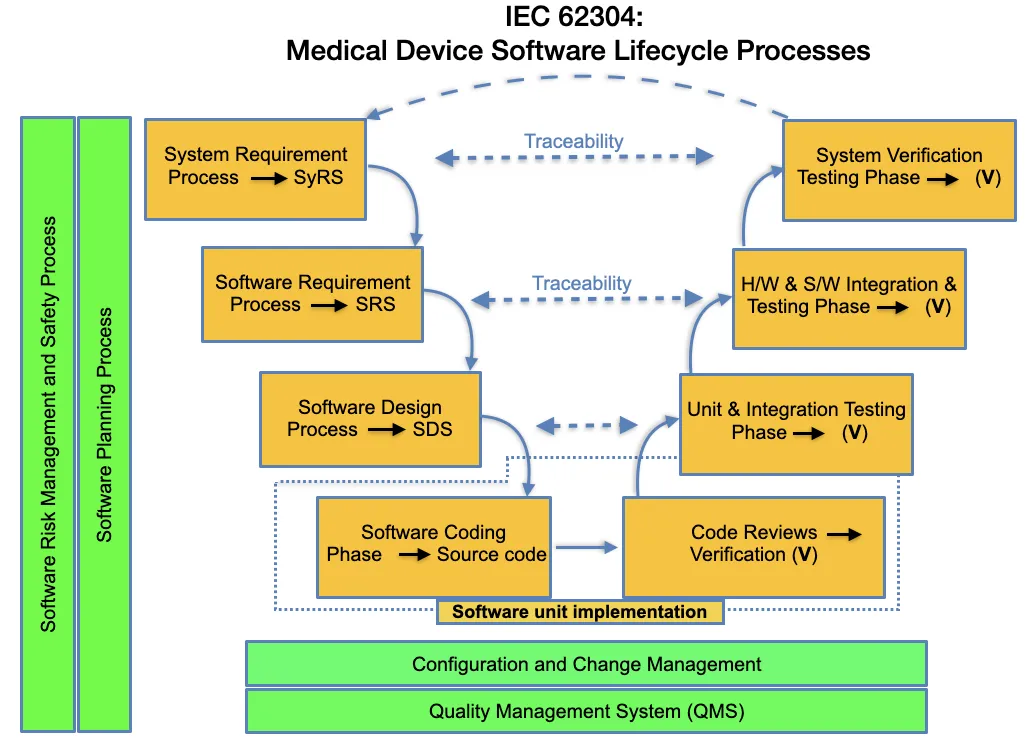
Importance of Software Lifecycle Management in Medical Devices
Now, let's explore the essential elements of an IEC 62304 checklist:
Quality Management System (QMS) Implementation
The foundation of IEC 62304 compliance is a robust Quality Management System (QMS). This system should encompass all aspects of software development and maintenance.
Key considerations:
Is there a documented QMS in place that covers software development processes?
Does the QMS align with other relevant standards like ISO 13485?
Are there clear procedures for document control, change management, and corrective actions?
Implementing a comprehensive QMS is crucial for IEC 62304 compliance and overall regulatory compliance and quality assurance. It serves as the central hub for all quality-related activities and documentation.
Software Safety Classification
IEC 62304 requires manufacturers to assign a safety class to their medical device software. This classification determines the level of rigor required in development and maintenance processes.
The three software safety classes are:
Class A: No injury or damage to health is possible
Class B: Non-serious injury is possible
Class C: Death or serious injury is possible
Key checklist items:
Has the software been assigned an appropriate safety class?
Is the classification documented and justified?
Are processes in place to reassess the classification if software functionality changes?
Proper classification is critical, as it impacts the scope of activities required for compliance. Misclassification can lead to either insufficient risk management or unnecessary overhead.
Software Development Planning
A well-defined Software Development Plan (SDP) is essential for IEC 62304 compliance. This plan outlines the software development lifecycle's processes, activities, and deliverables.
Checklist considerations:
Does a comprehensive SDP exist?
Does the plan cover all required IEC 62304 activities appropriate for the software's safety class?
Are roles and responsibilities clearly defined?
Does the plan include provisions for risk management and configuration management?
The SDP serves as a roadmap for the development team and provides evidence of a systematic approach to software development for auditors.
Software Requirements Analysis
Thorough requirements analysis ensures the software meets its intended use and safety requirements.
Key elements to check:
Are software requirements documented and traceable to system requirements to ensure complete traceability?
Do the requirements include both functional and non-functional aspects?
Has a risk analysis been performed on the requirements?
Is there a process for reviewing and approving requirements?
Well-defined requirements form the basis for subsequent development activities and help ensure that the final product meets user needs and regulatory expectations.
Software Architectural Design
The software architecture provides a high-level view of the software structure and its components. It's crucial for identifying and mitigating potential safety risks.
Checklist items:
Is there a documented software architecture?
Does the architecture identify software items and their interfaces?
Are safety-related components clearly identified?
Has the architecture been reviewed for potential safety implications?
A well-designed architecture can significantly impact the software's maintainability, scalability, traceability, and overall quality. It also plays a crucial role in risk management by helping to isolate safety-critical components.
Software Detailed Design
The detailed design specifies how each software component will be implemented based on the architectural design.
Key considerations:
Is there a documented detailed design for each software component?
Does the design provide sufficient information for implementation and testing?
Are interfaces between software units clearly defined?
Has the detailed design been reviewed for consistency with the architecture and requirements?
A thorough, detailed design helps ensure that implementation is consistent and all requirements are addressed.
Software Unit Implementation and Verification
This stage involves the actual coding of software units and verification that they meet their specified requirements.
Checklist items:
Is there a defined process for implementing software units?
Are coding standards in place and followed?
Is unit testing performed and documented?
Are code reviews conducted and documented?
Proper implementation and verification at the unit level can catch and resolve issues early, reducing the cost and effort required for later stages of testing and validation.
Software Integration and Integration Testing
Once individual software units are completed, they must be integrated and tested as larger components or systems.
Key elements to check:
Is there a defined integration strategy and plan?
Are integration tests documented and executed?
Are test results recorded and issues tracked?
Is there a process for regression testing when changes are made?
Effective integration testing helps ensure that different software components work together as intended and that the integrated system meets its requirements.
Software System Testing
System testing verifies that the complete software system meets its specified requirements and functions correctly in its intended environment.
Checklist considerations:
Is there a comprehensive system test plan?
Do test cases cover all software requirements?
Are test results documented and issues tracked?
Is there a process for addressing and retesting failed test cases?
Thorough system testing is crucial for identifying issues that may not have been caught during unit or integration testing and verifying overall system performance and reliability.
Software Release
The software release process ensures that only correctly verified and validated software is released for use.
Key items to check:
Is there a defined release process?
Are all required verification and validation activities completed before release?
Is there a process for creating and verifying release documentation?
Are released versions archived and retrievable?
A well-controlled release process helps prevent the distribution of software that may not meet quality or regulatory requirements.
Risk Management
Risk management is critical to IEC 62304 compliance and should be integrated throughout the software development lifecycle.
Checklist considerations:
Is there a documented risk management process for software?
Are potential hazards and hazardous situations identified and evaluated?
Are risk control measures implemented and verified?
Is the risk management file maintained and updated throughout the software lifecycle?
Effective risk management helps identify and address potential safety issues proactively, reducing the likelihood of harm to patients or users.
Configuration Management
Configuration management ensures that all software items, including source code, documentation, and development tools, are properly controlled and maintain high traceability.
Key elements to check:
Is there a defined configuration management process?
Are all configuration items uniquely identified and version-controlled?
Is there a process for managing changes to configuration items?
Are build processes documented and reproducible?
Proper configuration management is essential for maintaining control over the development process and ensuring that the correct software versions and documentation are used throughout the product lifecycle.
Problem Resolution
IEC 62304 requires a systematic approach to identifying, documenting, evaluating, and resolving software problems.
Checklist items:
Is there a defined process for problem reporting and resolution?
Are problems classified based on their potential impact on safety?
Is there a system for tracking problems and their resolution status?
Are problem reports and resolutions documented and retrievable?
An effective problem-resolution process helps ensure that issues are addressed promptly and systematically, reducing potential risks to patients and users.
Maintenance Process
Software maintenance is an ongoing process that continues throughout the product's lifecycle.
Key considerations:
Is there a defined maintenance process for released software?
Are maintenance activities planned and documented?
Is there a process for evaluating and implementing changes?
Are maintenance records kept and retrievable?
A well-defined maintenance process helps ensure the software remains safe and effective throughout its operational life.
Third-party Software Management
Many medical devices incorporate third-party or off-the-shelf software components. These need to be managed carefully to ensure overall system safety and compliance.
Checklist items:
Is there a process for evaluating and selecting third-party software?
Are the interfaces and behavior of third-party components well-documented?
Is there a process for managing updates to third-party software?
Are potential risks associated with third-party software identified and mitigated?
Proper management of third-party software helps ensure that it doesn't introduce unexpected risks or compliance issues into the overall system.
Implementing Your IEC 62304 Compliance Management System
While the checklist above covers the key elements of IEC 62304, implementing a comprehensive compliance management system requires a systematic approach. Here are some tips for success:
Integrate compliance into your development process: Rather than treating IEC 62304 compliance as a separate activity, integrate it into your existing software development lifecycle. This can help reduce overhead and ensure that compliance activities are performed consistently.
Use appropriate tools: Consider using specialized software tools for requirements management, risk analysis, and test management. These can help streamline compliance activities and provide better traceability.
Train your team: Ensure that all team members understand the requirements of IEC 62304 and their roles in maintaining compliance. Regular training sessions can help reinforce best practices.
Perform internal audits: Conduct regular internal audits to identify and address potential compliance gaps before they become issues during formal audits.
Stay up-to-date: Keep abreast of changes to IEC 62304 and related standards. The regulatory landscape continually evolves, and staying informed can help you adapt your processes accordingly.
Document everything: Maintain comprehensive documentation of all development activities, decisions, and rationales. This supports compliance and provides valuable information for future maintenance and updates.
Foster a culture of quality: Encourage all team members to take ownership of quality and compliance. A culture that values these aspects can lead to better outcomes and easier audits.
Conclusion
Implementing and maintaining compliance with IEC 62304 may seem daunting, but it's essential to developing safe and effective medical device software. By following the checklist outlined in this article and implementing a robust compliance management system, you can meet regulatory requirements and improve your software's overall quality and reliability.
Remember that compliance is an ongoing process, not a one-time event. Regularly reviewing and updating your processes, staying informed about regulatory changes, and fostering a culture of quality within your organization will help ensure long-term success in the medical device software industry.
By prioritizing IEC 62304 compliance, you're not just meeting a regulatory requirement – you're demonstrating a commitment to patient safety and product quality that can set your medical device apart in a competitive market. Whether you're a small startup or a large established manufacturer, investing in a comprehensive IEC 62304 compliance program is an investment in the future of your medical device software and, ultimately, in the well-being of the patients it serves.


