How To Master Food and Beverage Regulations and Compliance?
Feb 20, 2025
Operating within the food and beverage industry in the United States means working in one of the most heavily regulated environments.
Various government agencies, from the U.S. Food and Drug Administration (FDA) to the U.S. Department of Agriculture (USDA), impose rules that touch upon nearly every phase of production, distribution, and marketing.
Additional oversight at the state and local levels introduces further complexity.
While these regulations are in place to protect public health, maintain fair competition, and ensure consumers receive safe and accurately labeled products, navigating them can sometimes be a bit confusing.
The Regulatory Landscape: A Multi-Layered Web
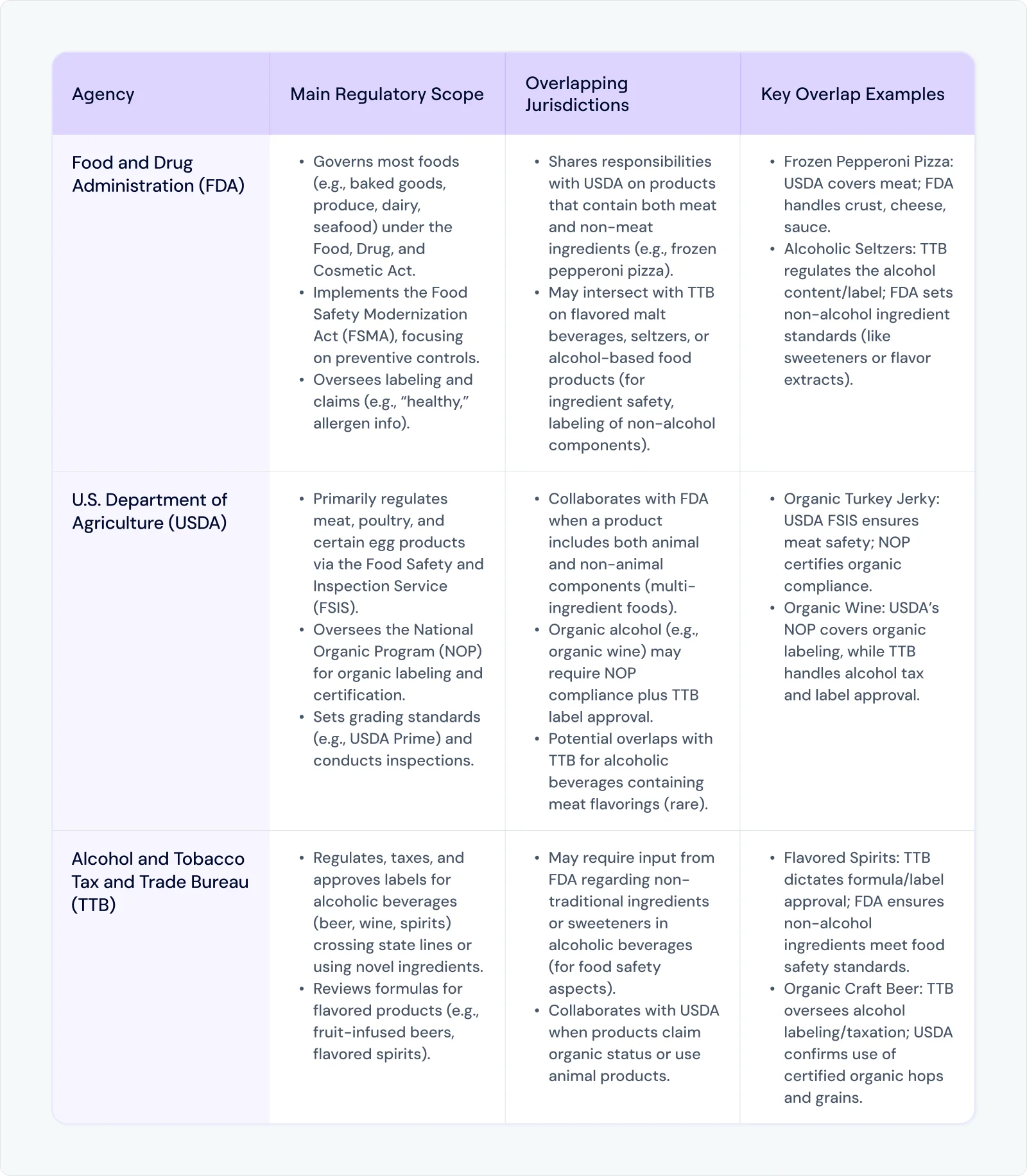
1. Federal Oversight: FDA, USDA, and TTB
At the federal level, three major agencies typically come into play for food and beverage businesses:
Food and Drug Administration (FDA) – Oversees most foods (baked goods, produce, dairy, seafood, and more) under the Food, Drug, and Cosmetic Act and, crucially, the Food Safety Modernization Act (FSMA).
U.S. Department of Agriculture (USDA) – Primarily regulates meat, poultry, and certain egg products through the Food Safety and Inspection Service (FSIS), while the National Organic Program (NOP) governs organic labeling and certification.
Alcohol and Tobacco Tax and Trade Bureau (TTB) – Responsible for regulating, taxing, and approving the labels of alcoholic beverages, particularly when crossing state lines or including novel ingredients.
However, each agency imposes standards that can overlap.
A single SKU – for instance, a frozen pepperoni pizza – may be subject to USDA rules (for meat) and FDA rules (for the crust, cheese, and sauce).
Adding complexity, label approval for alcoholic beverages through TTB might also intersect with state-specific distribution laws.
2. State-Level Regulations
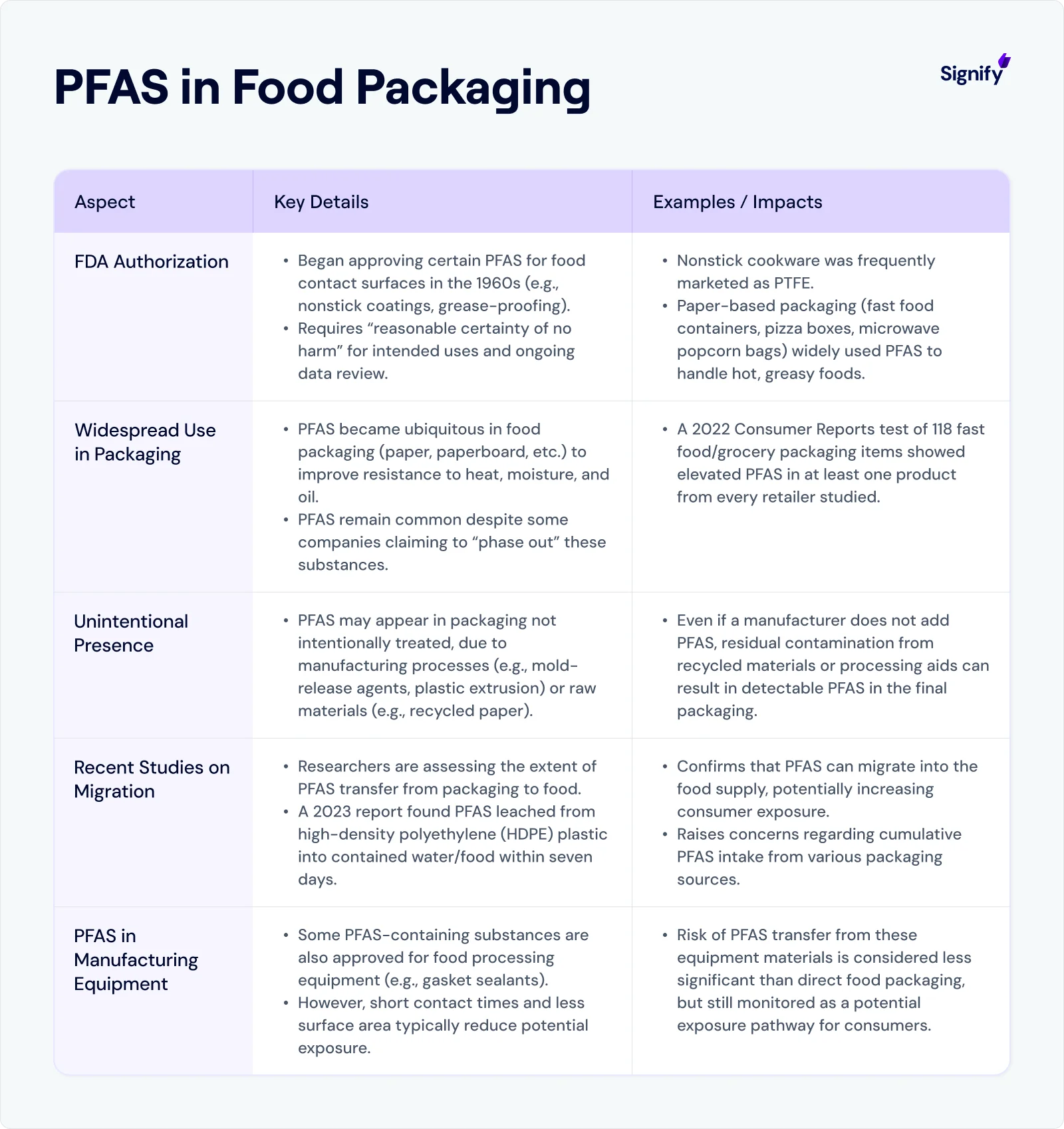
Individual states can impose additional requirements, often in the form of health department inspections, licensing standards, and restrictions on packaging chemicals.
California, for instance, has led the way in limiting the use of PFAS (“forever chemicals”) in food packaging, requiring businesses to adapt their materials or face restrictions on selling within the state.
3. Local Health Departments
At the local level, restaurants and food manufacturers often contend with periodic inspections that assess sanitation, temperature control, and hazard management.
The grading or scoring of establishments (e.g., displaying an “A” in the window) can significantly influence consumer perception – reinforcing that local compliance is not just a legal requirement but a business imperative for building trust.
Recent and Emerging Regulatory Developments
1. The Final Food Traceability Rule (FDA)
In November 2022, the FDA published its Final Food Traceability Rule, designed to bolster transparency and streamline recalls for high-risk foods (like fresh-cut fruits, leafy greens, eggs, and cheeses).
Although the official compliance date extends to 2026, businesses are encouraged to prepare early.
For example, incorporating digital traceability systems, refining supplier documentation, and training employees on new data-gathering requirements.
Key Takeaways:
Detailed digital records on ingredient movement and finished products will soon be expected.
In outbreak scenarios, companies should rapidly present the FDA with comprehensive traceability data.
Early adoption of modern tracking systems can reduce administrative burdens when the rule is fully enforced.
2. Proposed Updates to the “Healthy” Label (FDA)
The FDA is also revisiting the definition of “healthy” on food labels.
New guidelines (expected to become partially effective in 2025) would align the term more closely with Dietary Guidelines for Americans.
For instance, foods labeled as “healthy” may need to meet stricter limits for added sugars, sodium, and saturated fats and contain meaningful amounts of key nutrients.
Potential Impacts:
Reformulation may become necessary for brands that currently market products as healthy but exceed new thresholds in salt, sugar, or fat.
Label redesign and claims verification will be crucial, meaning extra label reviews and possibly laboratory testing for nutrient content.
3. USDA’s Organic Labeling Proposals
Simultaneously, the USDA is tightening rules around organic labeling, particularly related to imports.
In the past, some imported products have been incorrectly labeled organic due to fraudulent certificates.
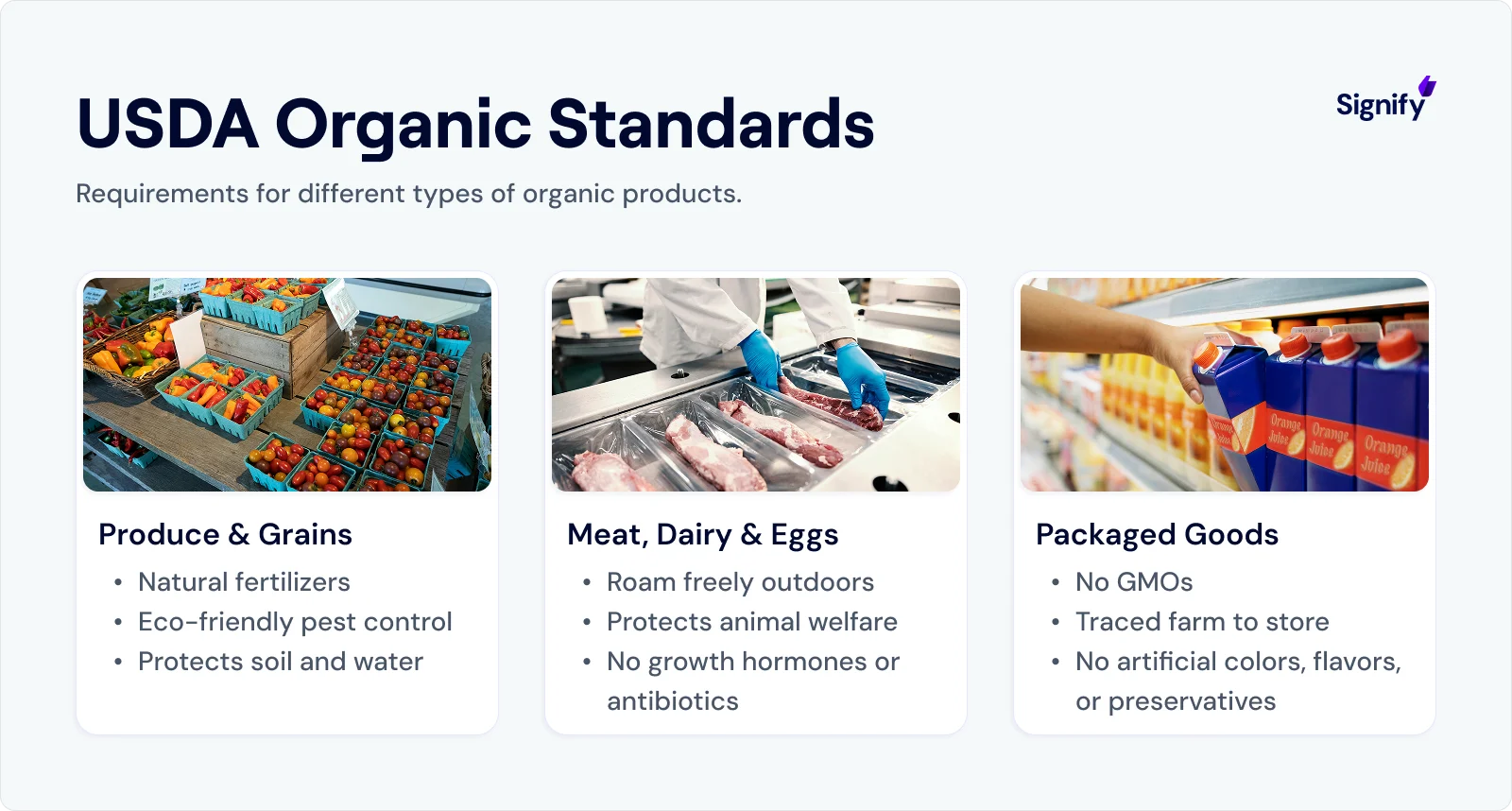
Under the proposed changes, businesses will need to show stronger documentation and may have to use USDA-accredited certifiers that meet enhanced training and oversight requirements.
Consequences for Importers:
Companies sourcing organic ingredients from overseas must vet foreign suppliers more rigorously.
There may be additional fees or extended timeframes for verifying organic status, so planning ahead is critical.
Once you’ve reformulated products and updated labels to meet the FDA’s stricter “healthy” benchmarks, ensure those changes also pass import and export scrutiny.
Pro Tip:
Signify can automatically verify that each revised label and shipping document complies with the FD&C Act, the Fair Packaging and Labeling Act, and any destination-specific laws.
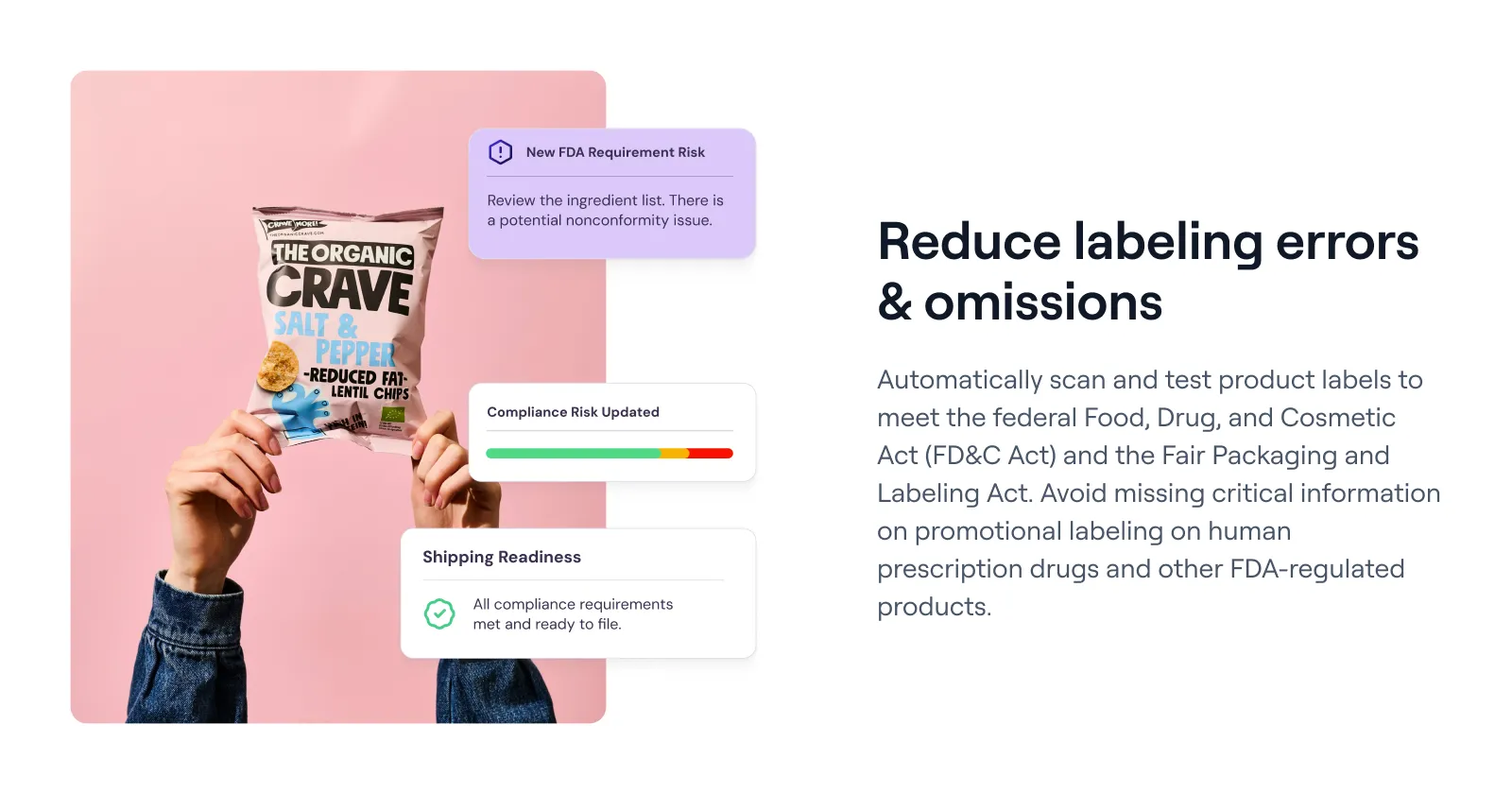
By catching omissions – such as missing local certifications or licensing details – Signify helps you avoid costly shipment holds at customs, safeguarding both your brand image and your bottom line across global markets.
4. TTB Modernization Initiatives
The TTB has recently explored ways to modernize label approvals and streamline formula submissions to keep up with the explosion of craft beverages (e.g., flavored hard seltzers, canned cocktails, and coffee-infused beers).
These changes aim to reduce the administrative burden on small producers while still ensuring labels meet consumer protection standards.
Common Compliance Hurdles
Despite ongoing modernization, food and beverage businesses still face a variety of challenges:
Regulatory Overlap: Managing multiple agency rules for one product.
Frequent Updates: Shifting definitions (like “healthy”) or new rules (like traceability requirements) demand constant vigilance.
Resource Constraints: Smaller companies may lack the capital to invest in advanced compliance tools or dedicated staff.
Global Complexity: Importing raw materials or exporting finished products entails verifying compliance with multiple jurisdictions.
Traceability Requirements: Keeping accurate, real-time records of every supply chain link is somehow difficult without robust technological solutions.
Practical Steps to Achieve Food and Beverage Industry Regulations and Compliance
1. Cultivate a Culture of Food Safety and Compliance
The first step is to foster an organizational mindset that sees compliance not as a necessary evil, but as a pillar of brand integrity.
Leadership should set the tone by emphasizing the importance of safety, funding relevant initiatives, and praising staff who proactively spot potential hazards.
2. Embrace HACCP and HARPC
To manage food safety systematically, most businesses rely on either HACCP (Hazard Analysis and Critical Control Points) or HARPC (Hazard Analysis and Risk-Based Preventive Controls).
HACCP is required for certain categories (like seafood and juice), while HARPC (under FSMA) applies to a broader range of FDA-regulated foods.
In essence, these frameworks help you identify potential hazards (biological, chemical, or physical) and monitor critical control points where contamination risks are highest.
HACCP: Required for certain categories (like seafood or juice) and focuses on pinpointing critical steps (e.g., cooking temperatures, allergen controls).
HARPC: An FSMA-driven system extending HACCP principles to a broader range of FDA-regulated products, adding a stronger emphasis on risk-based preventive measures (e.g., supplier verification and intentional adulteration prevention).
Key Components of HACCP/HARPC:
Hazard Analysis: Investigate what could go wrong at each production step.
Critical Control Points (CCPs): Determine points where hazards can be prevented or mitigated (e.g., cooking temperature, metal detector checks).
Monitoring & Verification: Regularly check and document that CCPs remain within safe limits.
Corrective Actions: Establish protocols for addressing deviations (e.g., discarding a batch, retraining employees).
3. Modernize Recordkeeping and Traceability
With the Final Food Traceability Rule on the horizon, digital traceability solutions are no longer optional.
Investing in cloud-based or blockchain-enabled systems can streamline data collection, reduce errors, and bolster your ability to manage recalls effectively.
Enterprise Resource Planning (ERP) platforms tailored for food and beverage can integrate inventory tracking, quality checks, and supplier documentation into one database.
Barcode/RFID Tagging allows for real-time scanning of ingredients and finished goods, aiding rapid identification of contamination surfaces.
Automatic Alerts can be set up so that any temperature deviation in storage triggers an immediate text or email to supervisors.
Cost Considerations
While top-tier ERP systems can be expensive for small operators, many scalable, cloud-based options now exist with monthly subscription models.
Additionally, some states and nonprofits offer grants or low-interest loans to help small businesses adopt traceability solutions, recognizing their public health benefit.
For additional info, check out our blog 8 Best Food Manufacturing Compliance Software in 2025.
4. Stay Proactive with Regulatory Updates
Regulations shift due to emerging science, public outcry (e.g., PFAS concerns), or policy changes. Staying ahead requires consistent monitoring of the following:
Federal Register notices for FDA, USDA, and TTB.
Trade association newsletters (e.g., Food Marketing Institute, National Restaurant Association) that summarize new or proposed rules.
Professional conferences focusing on food safety, which often provide previews of pending legislation or enforcement trends.
5. Conduct Internal and External Audits
Audits are not solely about meeting government inspections; they’re also vital for self-improvement.
Many businesses use a dual approach:
Internal Audits: Performed by a dedicated compliance or QA team, checking daily logs, SOP adherence, and staff training.
External Audits: Engaging third-party certifiers (e.g., SQF, BRC) to validate that your systems meet global standards. Passing these audits can unlock new markets or streamline your process during official inspections.
Pro Tip:
Don’t wait for an audit to uncover compliance issues – integrate real-time conformity assessments into your QMS.
Signify automatically scans for potential risks, tracks evidence for audits, and generates smart checklists, giving you instant visibility into compliance gaps.
This proactive approach keeps regulatory requirements top of mind, reduces the risk of product quality lapses, and strengthens customer trust by continually validating that your processes meet industry standards.
6. Strengthen Supplier Relationships
Suppliers are an extension of your operations, particularly if you’re importing ingredients. Under FSMA’s Foreign Supplier Verification Programs (FSVP) rule, importers are expected to verify that foreign suppliers meet U.S. safety standards.
Written Agreements should clarify quality expectations, testing protocols, and responsibility for recalls or contamination events.
Regular Communication ensures that you’re informed of any raw-material issues (e.g., pesticides in produce, antibiotic residues in dairy animals) before they escalate.
Audits or On-Site Visits offer a hands-on look at your supplier’s facilities, from their sanitation practices to how they label allergens.
Best Practices for Successful Implementation
Successfully navigating food and beverage regulations demands a holistic approach that weaves safety, transparency, and oversight into the fabric of daily operations.
1. Integrate Compliance into the Business Model
A business that treats regulations as an ongoing investment (rather than a one-time hurdle) tends to fare better in the long run.
Make compliance part of your value proposition, emphasizing that your products are produced under strict safety standards and rigorous quality checks.
2. Document Every Step But Maintain Readability
Long gone are the days when thick binders of poorly organized, handwritten records sufficed. Modern recordkeeping should be thorough yet easy to navigate. For example, you can use
a mix of digital logs and well-structured, standardized forms.
Because the system’s interface is intuitive, staff are more inclined to keep the information up-to-date and accurate.
During an FDA inspection, you can quickly retrieve logs for any date or product run, demonstrating real-time compliance.
3. Provide Regular, Engaging Training
One of the most common pitfalls is relying on annual “binge” trainings that employees quickly forget. Instead, continuous learning keeps everyone alert:
Short, Frequent Sessions: Five-minute refreshers at the start of each shift can cover critical points, like allergen labeling or temperature monitoring.
Hands-On Demonstrations: Show employees exactly how to calibrate a thermometer or clean a machine rather than simply telling them.
Real Scenarios: Use industry examples of outbreaks or recalls as teaching tools. Discuss how these incidents occurred, what went wrong, and how they could have been avoided.
4. Establish Clear Escalation Paths for Violations
When issues arise, like a positive Listeria test or an incorrect label, employees need to know whom to notify and what steps to take. Without a clear chain of command, valuable time is lost, and the problem can worsen.
5. Pursue Voluntary Certifications
Although not always mandated by law, certifications such as SQF (Safe Quality Food), BRC Global Standard, or FSSC 22000 demonstrate a commitment to globally recognized best practices. Large retailers often prefer or even require these certifications because they reduce the risk of stocking unsafe or non-compliant products.
Key Benefits:
Streamlined Inspections: When inspectors see that you’ve met a GFSI (Global Food Safety Initiative) benchmark, they often trust that your systems are robust.
Market Access: These certifications can open up new distribution channels—particularly if you plan to sell internationally.
6. Plan for New Markets and Emerging Trends
If you aim to expand into alcoholic beverages or decide to produce plant-based “meats,” research the specialized requirements well in advance.
The TTB imposes formula approvals for unique beer and spirit recipes, and the USDA is currently finalizing regulations for labeling cell-cultivated meat.
Understanding these niche rules helps you avoid delays or rejections when applying for label approvals.
Conclusion
Overcoming food and beverage industry regulations in the U.S. is undeniably challenging, but it’s also an arena ripe with opportunity.
As new rules emerge, forward-thinking companies will invest in the systems, training, and culture required not just to meet these standards but to surpass them.
A robust, technology-driven approach to traceability and documentation can reduce recall risks, build consumer trust, and even differentiate your products in a crowded marketplace.
How Can Signify Transform Your Food and Beverage Compliance?
Signify is an AI-driven regulatory compliance automation platform crafted to streamline food safety, regulatory adherence, and risk management.
By automating key processes and delivering real-time insights, Signify enables food manufacturers to stay audit-ready and adapt rapidly to regulatory changes, all while minimizing manual efforts.
Key Benefits of Using Signify
Proactive Compliance Monitoring: Signify’s AI engines analyze SOPs, labels, and supplier documentation in real-time, pinpointing any regulatory inconsistencies before they evolve into non-conformities.
Smart Documentation Management: Automated linking and organizing of all compliance records streamlines audit readiness and creates a clear documentation trail – perfect for traceability and reporting.
Regulatory Risk Intelligence: Stay informed about shifting regulations with instant conformity checks. Signify flags potential risk factors as they emerge so you can address issues promptly.
Automated Compliance Testing: The platform’s AI continuously evaluates your compliance data, ensuring risks are caught early, and non-conformities are minimized or prevented altogether.
Remediation Guidance: When gaps arise, Signify provides practical compliance remediation actions, helping you course-correct swiftly and maintain alignment with evolving food safety rules.
Ready to Elevate Your Compliance Efforts?
Book a demo to experience how Signify simplifies regulatory automation, mitigates risk, and ensures food safety – all in one unified platform.
